What Is The Bohr Diagram For Magnesium . Commence by sketching the nucleus, and then illustrate the three electron shells. In the nucleus you would show the 12 neutrons and 12 protons. To draw the magnesium bohr model, represent the 12 protons, 12 neutrons, and 12 electrons. Group 18 elements (helium, neon, and argon are shown) have a full. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. We’ll use a bohr diagram to visually represent where the electrons are around. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Bohr diagrams indicate how many electrons fill each principal shell. So far, 10 of magnesium's 12 electrons have been used, so. Atoms are way too small to see with the naked eye. Learn how bohr models are used to represent atoms. In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). Magnesium has 3 energy levels: The bohr model and atomic spectra. Magnesium has 12 protons and 12 electrons.
from byjus.com
The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. The bohr model and atomic spectra. In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). Group 18 elements (helium, neon, and argon are shown) have a full. In the nucleus you would show the 12 neutrons and 12 protons. Learn how bohr models are used to represent atoms. So far, 10 of magnesium's 12 electrons have been used, so. We’ll use a bohr diagram to visually represent where the electrons are around. The first electron shell of a bohr model holds 2 electrons. Commence by sketching the nucleus, and then illustrate the three electron shells.
According to Bohr orbital theory, the angular momentum of electron in
What Is The Bohr Diagram For Magnesium Magnesium has 12 protons and 12 electrons. In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). The first electron shell of a bohr model holds 2 electrons. We’ll use a bohr diagram to visually represent where the electrons are around. Learn how bohr models are used to represent atoms. Group 18 elements (helium, neon, and argon are shown) have a full. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. To draw the magnesium bohr model, represent the 12 protons, 12 neutrons, and 12 electrons. In the nucleus you would show the 12 neutrons and 12 protons. Bohr diagrams indicate how many electrons fill each principal shell. Commence by sketching the nucleus, and then illustrate the three electron shells. The bohr model and atomic spectra. So far, 10 of magnesium's 12 electrons have been used, so. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Magnesium has 12 protons and 12 electrons. Atoms are way too small to see with the naked eye.
From valenceelectrons.com
Magnesium Electron Configuration Aufbau & Bohr Model What Is The Bohr Diagram For Magnesium Group 18 elements (helium, neon, and argon are shown) have a full. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. In the nucleus you would show the 12 neutrons and 12 protons. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. We’ll use. What Is The Bohr Diagram For Magnesium.
From imgbin.com
Magnesium Chemical Element Bohr Model Diagram PNG, Clipart, Area, Atom What Is The Bohr Diagram For Magnesium 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. The first electron shell of a bohr model holds 2 electrons. Bohr diagrams indicate how many electrons fill each principal shell. To draw the magnesium bohr model, represent the 12 protons, 12 neutrons, and 12 electrons. In this video we'll look at. What Is The Bohr Diagram For Magnesium.
From flyclipart.com
Acs Logo Bohr Diagram For Magnesium, Urban, Text, City HD PNG Download What Is The Bohr Diagram For Magnesium We’ll use a bohr diagram to visually represent where the electrons are around. Magnesium has 3 energy levels: The bohr model and atomic spectra. To draw the magnesium bohr model, represent the 12 protons, 12 neutrons, and 12 electrons. Bohr diagrams indicate how many electrons fill each principal shell. The bohr model of magnesium (mg) has a nucleus that contains. What Is The Bohr Diagram For Magnesium.
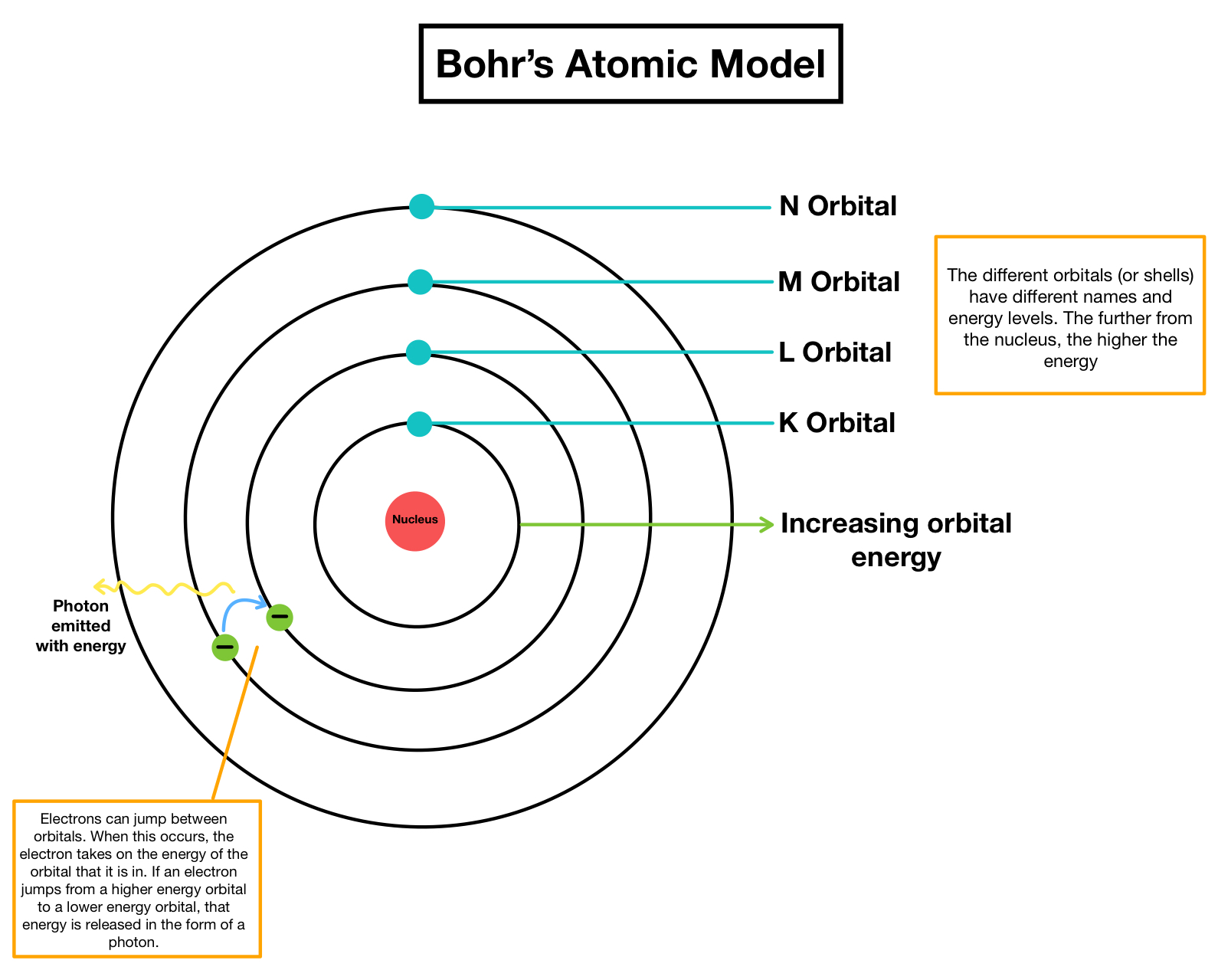
From www.tec-science.com
Bohr's atomic model tecscience What Is The Bohr Diagram For Magnesium In the nucleus you would show the 12 neutrons and 12 protons. Group 18 elements (helium, neon, and argon are shown) have a full. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). Learn how bohr. What Is The Bohr Diagram For Magnesium.
From brainly.in
Diagramatic sketch of electronic configuration of magnesium (atomic What Is The Bohr Diagram For Magnesium Commence by sketching the nucleus, and then illustrate the three electron shells. The first electron shell of a bohr model holds 2 electrons. Learn how bohr models are used to represent atoms. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. Magnesium has 12 protons and 12 electrons. Magnesium has 3. What Is The Bohr Diagram For Magnesium.
From www.cartoongames.online
Bohr Atomic Model Formula, Postulates and Limitations, Diagram What Is The Bohr Diagram For Magnesium Bohr diagrams indicate how many electrons fill each principal shell. The bohr model and atomic spectra. Atoms are way too small to see with the naked eye. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. Commence by sketching the nucleus, and then illustrate the three electron shells. Learn how bohr. What Is The Bohr Diagram For Magnesium.
From axiom-northwest.com
Magnesium Bohr Diagram What Is The Bohr Diagram For Magnesium In the nucleus you would show the 12 neutrons and 12 protons. Magnesium has 3 energy levels: So far, 10 of magnesium's 12 electrons have been used, so. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Group 18 elements (helium, neon, and argon are shown) have a full. In this video we'll. What Is The Bohr Diagram For Magnesium.
From www.aiophotoz.com
Magnesium Chemical Element Bohr Model Diagram Png 556x569px Images What Is The Bohr Diagram For Magnesium Commence by sketching the nucleus, and then illustrate the three electron shells. Magnesium has 12 protons and 12 electrons. Group 18 elements (helium, neon, and argon are shown) have a full. The bohr model and atomic spectra. In the nucleus you would show the 12 neutrons and 12 protons. Learn how bohr models are used to represent atoms. Bohr diagrams. What Is The Bohr Diagram For Magnesium.
From cabinet.matttroy.net
Properties Of Magnesium On The Periodic Table Matttroy What Is The Bohr Diagram For Magnesium To draw the magnesium bohr model, represent the 12 protons, 12 neutrons, and 12 electrons. Atoms are way too small to see with the naked eye. Commence by sketching the nucleus, and then illustrate the three electron shells. Magnesium has 3 energy levels: In the nucleus you would show the 12 neutrons and 12 protons. In this video we'll look. What Is The Bohr Diagram For Magnesium.
From userengineunstrap.z14.web.core.windows.net
Bohr Diagram Of Na What Is The Bohr Diagram For Magnesium In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). So far, 10 of magnesium's 12 electrons have been used, so. We’ll use a bohr diagram to visually represent where the electrons are around. The bohr model and atomic spectra. The first electron shell of a bohr model holds 2 electrons. Commence by. What Is The Bohr Diagram For Magnesium.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background What Is The Bohr Diagram For Magnesium Atoms are way too small to see with the naked eye. In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). Group 18 elements (helium, neon, and argon are shown) have a full. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. The first electron shell. What Is The Bohr Diagram For Magnesium.
From mungfali.com
Magnesium Bohr Model What Is The Bohr Diagram For Magnesium Group 18 elements (helium, neon, and argon are shown) have a full. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. The first electron shell of a bohr model holds 2 electrons. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. Magnesium has 3. What Is The Bohr Diagram For Magnesium.
From www.americanelements.com
Magnesium (Mg) AMERICAN ELEMENTSs What Is The Bohr Diagram For Magnesium In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). Atoms are way too small to see with the naked eye. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. To draw the magnesium bohr model, represent the 12 protons, 12 neutrons, and 12. What Is The Bohr Diagram For Magnesium.
From byjus.com
According to Bohr orbital theory, the angular momentum of electron in What Is The Bohr Diagram For Magnesium The first electron shell of a bohr model holds 2 electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. So far, 10 of magnesium's 12 electrons have been used, so. Group 18 elements (helium, neon, and argon are shown) have a full. In the nucleus you would show the 12 neutrons and. What Is The Bohr Diagram For Magnesium.
From www.youtube.com
Bohr Diagram for Magnesium Satisfying the Octet Rule YouTube What Is The Bohr Diagram For Magnesium Magnesium has 12 protons and 12 electrons. Commence by sketching the nucleus, and then illustrate the three electron shells. In the nucleus you would show the 12 neutrons and 12 protons. In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). We’ll use a bohr diagram to visually represent where the electrons are. What Is The Bohr Diagram For Magnesium.
From mavink.com
Magnesium Bohr Model Diagram What Is The Bohr Diagram For Magnesium The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Learn how bohr models are used to represent atoms. The bohr model and atomic spectra. To draw the magnesium bohr model, represent the 12 protons, 12 neutrons, and 12 electrons. Magnesium has 3 energy levels: Atoms are way too small to see with the. What Is The Bohr Diagram For Magnesium.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image What Is The Bohr Diagram For Magnesium To draw the magnesium bohr model, represent the 12 protons, 12 neutrons, and 12 electrons. The bohr model and atomic spectra. In the nucleus you would show the 12 neutrons and 12 protons. Learn how bohr models are used to represent atoms. Group 18 elements (helium, neon, and argon are shown) have a full. 2 electrons in the 1st level,. What Is The Bohr Diagram For Magnesium.
From www.elektramagnesium.com.au
Ionic Magnesium Chloride Hexahydrate or Chelated Magnesium? Elektra What Is The Bohr Diagram For Magnesium The first electron shell of a bohr model holds 2 electrons. We’ll use a bohr diagram to visually represent where the electrons are around. The bohr model and atomic spectra. Atoms are way too small to see with the naked eye. Learn how bohr models are used to represent atoms. Magnesium has 12 protons and 12 electrons. In the nucleus. What Is The Bohr Diagram For Magnesium.